SO2 is one of the highly toxic gases, which poisons humans by inhalation. Sulfur dioxide is released when compounds containing sulfur, such as fossil fuels like coal are burned. Nanocrystalline SnO2 thin film based portable sensor detects SO2 from 2 ppm onwards at room temperature with fast response and recovery. The sensor works on the principle of decrease in electrical resistance upon a chemical interaction with SO2.
Commercially available SO2 sensors are usually operated at high temperature to achieve reversibility and fast response. Shortcomings of high temperature operation of these sensors are: shorter life time, does not have portability and is not favorable in explosive environment.
Gas sensors are usually operated at high temperature to achieve fast response and reversibility and this leads to shorter lifetime of the sensor. Nanocrystalline SnO2 thin films fabricated from the thermal decomposition of Langmuir Blodgett (LB) film precursor, exhibit room temperature gas sensitivity comparable to that required for air quality monitoring. LB technique offers control over SnO2 film thickness and crystallite size. By controlling the crystallite size and film thickness room temperature operation is achieved. The sensor is specific to SO2 gas at room temperature and shows fast response and recovery without any carrier gas flow. The stability studies indicated that these sensors are stable at least for a year with no significant change in sensitivity.
| Sr. No. | Specification | Value |
|---|---|---|
| 1 | SO2 detection range | 1-30 ppm |
| 2 | Operating temperature | Room temperature |
| 3 | Safe operating temperature | 25-50°C |
| 4 | Response time (time to reach 90% of the resistance change) | 50 sec |
| 5 | Recovery time (time to reach 10% of the resistance change) | 30 min |
| 6 | Response at 2 ppm SO2 [(Rair - Rgas)x100]/Rair | 80-100% |
| 7 | Selectivity for SO2 (in comparison to CH4, CO, NH3, NO2, H2 etc.) | Detectable increase in current only for SO2 among these gases |
| 8 | Life time under operating conditions | ~ 1 year |
SO2 is one of the highly toxic gases, which poisons humans by inhalation. SO2 combines with water to form sulfuric acid and can irritate the throat by inhalation. Sulfur dioxide is released when compounds containing sulfur, such as fossil fuels like coal are burned. Its long-term (8 h) and short-term (10 minutes) exposure limits are 2 and 5 ppm respectively. The nanocrystalline SnO2 thin film based sensor detects SO2 with high sensitivity, selectivity, and is stable for more than 1 year. Commercially available SnO2 gas sensors are usually operated at high temperature to achieve reversibility and fast response. High temperature operation causes shorter life time and not favorable in explosive environment.
The sensor works on the principle of decrease in electrical resistance upon a chemical interaction with SO2. This change is calibrated and displayed directly on a digital monitor as SO2 concentration in ppm.
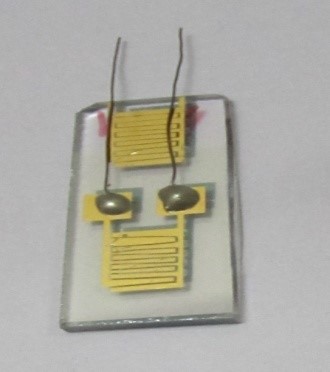
Preparation of SnO2 thin film and sensing mechanism :
The sensor element is nanocrystalline SnO2 thin film which is prepared by using Langmuir- Blodgett (LB) film precursors. Multilayer LB films (100 ± 20 layers) of ODA–stannate complex deposited on quartz are decomposed at 600 °C in the presence of ambient oxygen.
Nanocrystalline SnO2 surface has net negative charge due to the large number of surface species O2-,O- present on the SnO2 surface. SO2 is a strong reducing gas. When SO2 gas is injected into the gas testing chamber containing SnO2 thin film, it reacts with surface oxygen species ( O2-,O-) forming SO3 while releasing electrons to the SnO2 conduction band, thereby decreasing the resistance. Ambient oxygen subsequently chemisorbs on the oxygen vacant SnO2 surface attracting electrons from SnO2 conduction band during the recovery phase. This makes the sensor specific to sulfur dioxide at room temperature.
| Sr. No. | Property | Value |
|---|---|---|
| 1 | Drift in base current | 2-3 % |
| 2 |
Sensitivity (towards SO2) S=((Rair-RSO2)x 100)/Rair |
80-100 % at 2 ppm of SO2 |
| 3 | Response time | 50 sec |
| 4 | Recovery time (after opening of stopcocks, without any carrier gas flow) | 30 min for 90 % recovery |
| 5 | Range of the sensor | 1-30 ppm |
| 6 | Repeatability | 90% |
| 7 | Percentage error in sensitivity | 10-15% |
| 8 | Sensor Housing | Water and light proof |
Selectivity in presence of other gases
| Selectivity and Sensitivity (as change in current ) | ||
|---|---|---|
| Gas | Change in current | Gas concentration |
| NH3 | decrease | 1 ppm |
| H2 | no change | 6 ppm |
| H2O | no change | < 97% RH |
| CO | no change | 6 ppm |
| H2S | increase | > 1 ppm (interfering gas) |
| SO2 | increase | > 1 ppm |
| NO2 | decrease (with recovery only after heating at 70°C for 2 hours) | > 85 ppb |
Conclusion
Sensor is highly selective for SO2 in presence of other gases (except for H2S gas) as mentioned above in the table.
Raw materials
Equipments
Space
2 Numbers