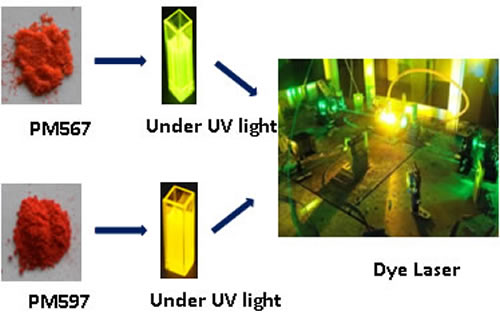
A gain medium is needed for generating Laser Beam in visible/ non visible spectrum. Some of the common mediums used are Ruby, CO2, Nd:YAGetc. Advantage of Dye Laser technology is that we can create an active medium by dispersing this highly fluorescent Dye in liquids, glass, polymer etc. also and generate tunable laser wavelengths, generally in the visible spectrum. The process for synthesis of PM 567 and PM 597 has been developed which are suitable gain medium for dye Laser action in visible spectral range 550-590 nm.
Process technology for synthesising efficient Pyrromethene (PM) laser dyes such as PM567 and PM597 has been developed.These are useful for high average power and high repetition rate dye lasers. Also,PM dyes are potential candidates to be used as chemosensors, bio-labelling and in photodynamic therapy.
A gain medium in an optical resonator cavity is needed for generating Laser Beam in visible/ non visible spectrum. Some of the common mediums, solid, liquid or gas, used are Ruby, CO2, CVL, Nd:YAG. Ti:Sa etc. Advantage of Dye Laser technology is that we can devise a gain medium by dispersing this highly fluorescent dye in liquids, glass, polymer etc. also and generate tunable laser wavelengths, generally in the visible spectrum.
Process technology for synthesising efficient BODIPY or Pyrromethene (PM) laser dyes such as PM567 and PM597 has been developed. These PM dyes are efficiently used in high repetition rate (~10 kHz), high average power dye lasers. Also, PM dyes are potential candidates to be used as chemosensors, bio-labelling and in photodynamic therapy.
Preamble
Despite existing for almost a century, fluorescent dyes continue to attract the attention of scientists from an ever expanding multidisciplinary arena. Recent developments in the field of personal diagnostics and in the area of organic electroluminescent devices have boosted interest in the development of next-generation emissive dyes. Countless classes of highly fluorescent organic compounds are now known, but the difluoro-boraindacene family (4,4-difluoro-4-borata-3a-azonia-4a-aza-s-indacene, abbreviated hereafter as BODIPY, also called Pyrromethene) has gained recognition as being one of the more versatile fluorophores and steadily increased in popularity over the past two decades [1]. The first member of this class of compound was reported by Treibs and Kreuzer in 1968 [2], although relatively little attention was given to the discovery until the end of the 1980s [3]. During the late 1980s and early 1990s, Boyer and co-workers established BODIPY dyes as a new class of laser dyes with emission covering the spectral region from the green yellow to the red [4]. Their high fluorescence yields in conjunction with excellent lasing efficiencies, intense absorption profiles in the green spectral region and low triplet–triplet extinction coefficients over the entire fluorescence region are the major attractions for the dye laser scientist [5]. These are zwiter-ionic dyes exhibiting good solubility in many organic solvents and even in methyl methacrylate (MMA) that is useful for solid state dye laser applications [6a-d]. Some of the Pyrromethene (PM) dyes outperform the widely used laser dye, rhodamine 6G (Rh6G), considered as the benchmark in lasing efficiency and photochemical stability.
Subsequently, the potential use of these dyes for biological labelling was recognized [6], and several new PM-based dyes were designed and even commercialized for biological application . As a consequence, PM dyes came to be known to the biochemist and biologist as a photostable substitute for fluorescein, and the number of papers and patents started to escalate in the mid 1990s. The use of PMs as effective biological label has been complemented by their propensity to function as a tunable laser dye.
The basic unit of the PM dye is pyrrole which is condensed with suitable acid chloride or aldehyde to form dipyrromethene. The complexation of a dipyrromethene unit to a boron trifluoride salt lead to the formation of the dipyrrometheneboron difluoride structure, which can be considered as being an example of a “rigidified” monomethine cyanine dye. The greatly restricted flexibility leads to unusually high fluorescence yields from the dipyrromethene-boron framework. Conjugation of the p-electrons runs along the organic backbone and can be extended by attachment of suitable groups onto the periphery or to one or both pyrrole fragments.
Brief description of Pyrromethene 567 and Pyrromethene 597
Process development of widely used, highly fluorescent and photo-chemically stable laser dyes – Pyrromethene 567 (PM567) [7] and Pyrromethene 597 (PM597) [7] belongs to PM dye family, in high purity (>99%) and good yield, are proposed for technology transfer. The functional use of this relatively recent fluorescent molecule, substituting Xanthene class dyes, will increase in next decades.
Researchers and technologists have been using these relatively recent laser dyes as gain media in dye lasers which are operated at high-repetition-rate (>10 KHz), high average power, as well as, low-repetition-rate (10-100 Hz), high peak intensity (several MW/cm2). These fluorescent molecules and their functionalized derivatives have great potential to use as chemosensors, biolabeling and in photodynamic therapy for effective generation of singlet oxygen. A few shortcomings of the commercially available laser dye sample are also addressed in order to improve their performance characteristics such as purity, solubility, quantum yield of fluorescence, lasing efficiency and photochemical stability.
This highly fluorescent Pyrromethene dye is widely used as gain medium in the constructed high average power, narrow line width dye lasers. In addition, many dye laser labs, biological and chemical labs in various research institutes in India have been using this costly fluorescent dye through import. This dye is dissolved in ethanol, methanol or n-heptane solvent and used in high power dye lasers as gain medium to obtain efficient and sustainable dye laser action in the visible spectral range 550-610 nm. These dye lasers may be pumped by second harmonic of Nd-YAG or diode pumped solid state laser (at 532 nm) or green output (at 511 nm) of Copper Vapour lasers (CVL). The Perennial problem of rapid photochemical degradation of these dyes in liquid dye lasers are substantially reduced using additive DABCO and/or purging of N2 in alcohol or n-heptane solvent. These fluorescent molecules and their functionalized derivatives have great potential to use as cation/anion chemosensors, biolebelling and in photodynamic therapy for effective generation of singlet oxygen.
INFRASTRUCTURE
MANPOWER
Chemicals and solvents
Quality of the product developed
The comparative laser performances of the developed products were evaluated with the imported product and shown below, which establishes superior quality of the locally developed dye sample.
Table 1: Comparative laser characteristics of commercial and synthesized PM 567 dye
| No. | Parameters | Exciton Sample | BARC sample | Remark |
|---|---|---|---|---|
| 1 | Absorption maxima (longest wavelength) | 517 nm | 517 nm | similar |
| 2 | Fluorescence Spectra (excitation at 490nm) | 526 nm | 526 nm | similar |
| 3 | Relative quantum yield of Fluorescence (QYF) | 0.84 | 0.84 | similar |
| 4 | Narrow band lasing performance (pump-Nd:YAG at 532nm) | Peak Efficiency (at λ =558.3nm): 14.7% | Peak Efficiency (at λ =558.3nm) : 15.2% | comparable |
| 5 | Photochemical stability (photons/molecule) | 2.1 X 103 (at 5mJ/pulse pump energy) | 2.9 X 103 (at 5mJ/pulse pump energy) | comparable |
| 6 | Purity (By HPTLC) | 96±1 % | 95±1 % | comparable |
| 7 | Physical appearance | Red, Crystalline | Red, Crystalline | comparable |
Table 2: Comparative laser characteristics of commercial and synthesized PM 597 dye.
| No. | Parameters | Exciton sample | BARC sample | Remark |
| 1 | Absorption maxima (longest wavelength) | 524±1 nm | 524±1 nm | similar |
| 2 | Fluorescence Spectra (excitation at 490nm) | 557±1nm | 557±1nm | similar |
| 3 | Relative quantum yield of Fluorescence (QYF) | 0.45 | 0.47 | similar |
| 4 | Narrow band lasing performance (pump-Nd:YAG at 532nm) | Peak Eff.(at λ =558.3nm): 18.8% | Peak Eff. (at λ =558.3nm): 17.0% | simillar |
| 5 | Purity (By HPTLC) | 97±1 % | 98±1 % | comparable |
| 6 | Physical appearance | Red, Crystalline | Red, Crystalline | comparable |
References